Welcome to FUNKI
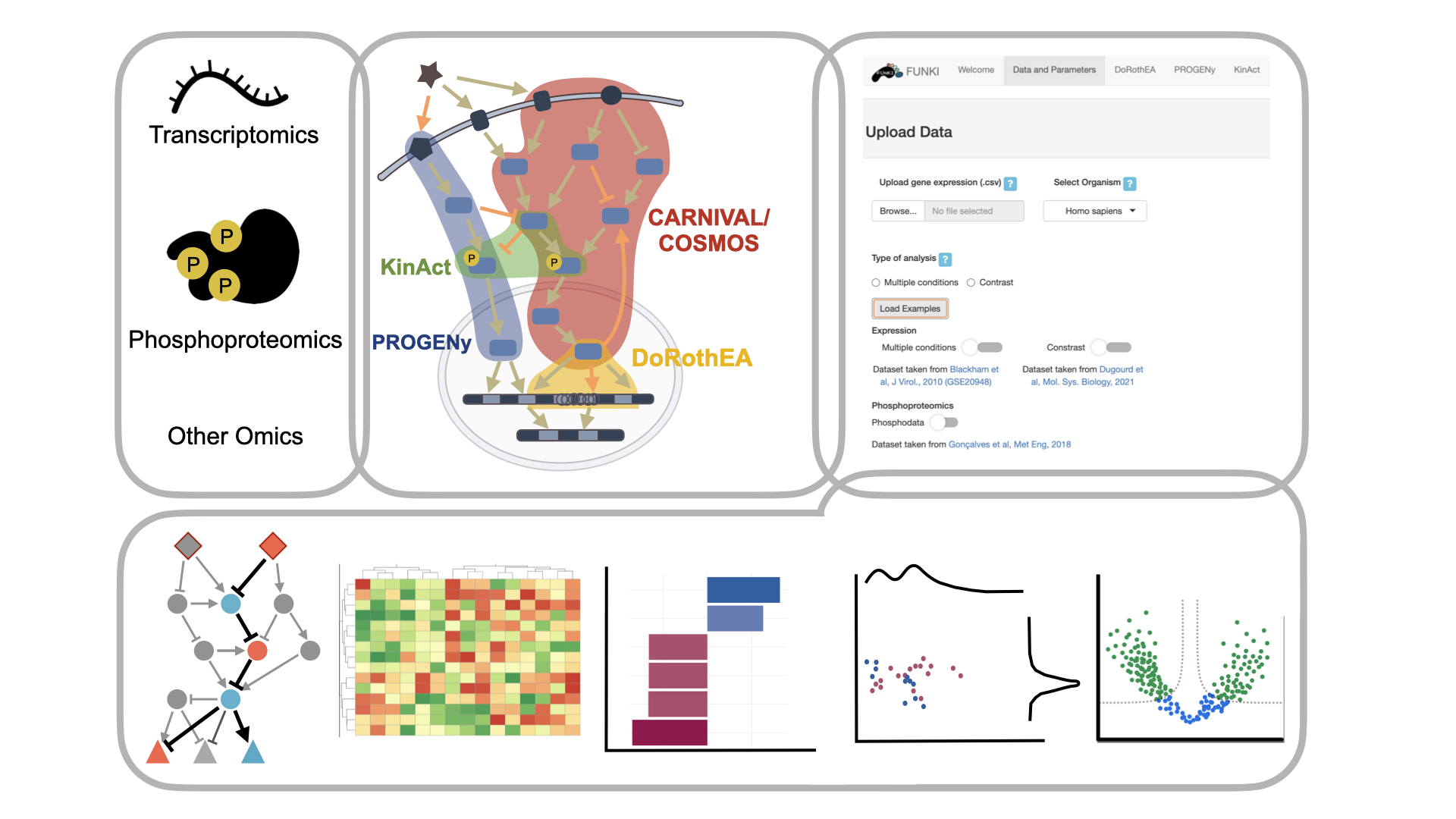
FUNKI is a multi-omic functional integration and analysis platform. It provides a standardised pipeline to process and perform functional analysis on transcriptomic, proteomic, phosphoproteomic and metabolomic datasets. The analysis can be performed both on a single type of omic data and on multi-omic dataset by integrating them in supervised and unsupervised manners.
Installation
Please find here instructions how to install FUNKI.
Implemented Approaches
DoRothEA
DoRothEA (Discriminant Regulon Expression Analysis) is a resource that links transcription factors (TFs) with their downstream targets ( Garcia-Alonso et al., 2018, 2019 ). The unity of a TF and its targets is called regulon. The regulons are built from four different strategies: (i) manually curated interaction repositories, (ii) interactions derived from ChIP-seq binding data, (iii) in silico predictions of TF binding on gene promoters, and (iv) reverse-engineered regulons from gene expression datasets. The TFs activities are computed from gene expression by performing an enrichment analysis ( Alvarez et al., 2016 ), where the regulons are the underlying gene-sets. The users can select the confidence level (A to E based on the type of the supporting evidence of given interactions) for each regulon, as well as their minimum size and the method to perform the enrichment analysis. The organism selection, human or mice, is selected when the data are uploaded ( Holland, Szalai, et al., 2020 ). This method can also be used with single-cell data ( Holland, Tanevski, et al., 2020 ). The computation yields a matrix with the normalised enrichment scores for each TF across all samples. This result is then visualised in the form of a heatmap, barplots and a network showing a TF with all its targets. Please find here the Tutorial.
PROGENy
PROGENy (Pathway RespOnsive GENes) is a footprint method developed to infer pathway activity from gene expression data (Schubert et al., 2018). The scores are calculated using a linear models with weights based on consensus gene signatures obtained from publicly available perturbation experiments. This method can be used for either bulk or single-cell data Holland, Tanevski, et al., 2020 from human or mouse ( Holland, Szalai, et al., 2020 ). They can also select the number of top genes from the signatures according to their individual significance. This last parameter is particularly important for the single-cell data as it counteracts the typical low gene coverage of this data type. PROGENy returns a matrix of pathway activity scores across all samples. This result is then visualized as a heatmap, barplot and density-scatter plot. Please find here the Tutorial.
KinAct
KinAct is a resource linking kinases to phosphorylation sites ( Wirbel et al., 2018 ). It is fully integrated into Omnipath due to the addition of kinase-substrate interaction resources ( Türei et al., 2021, 2016 ). Kinase activity estimation is performed using the same algorithm as DoRothEA to estimate activity scores ( Alvarez et al., 2016 ). Instead of TF-target interactions, KinAct uses collections of kinase-substrate interactions and phosphoproteomic data instead of transcriptomic data. The users can select the minimum size of each regulon, as well as the method that VIPER will use to perform the analysis. The result is a matrix of normalised enrichment scores for each phosphosite across all samples. This result is then visualised in the form of a heatmap, barplots and a network showing a kinase with the targeted phosphosites. Please find here the Tutorial.
CARNIVAL
CARNIVAL (CAusal Reasoning for Network identification using Integer VALue programming) reconstructs signalling networks from downstream TF activities by finding the upstream regulators ( Liu et al., 2019 ). Given a directed prior-knowledge network (PKN) of protein-protein interactions, which can also be signed, CARNIVAL identifies a subnetwork that explains the activities of transcription factors through potential perturbed intermediate genes. The PKN can be provided by the user, or imported from Omnipath directly within FUNKI ( Türei et al., 2016, 2016 ). Similarly, the user can directly upload the activity of transcription factors, but those can also be calculated using DoRothEA from the expression data. When large initial networks are used, mapping key nodes with values is advised. Thus, the user can upload them or get advantage of the PROGENy scores for this task. As the previous methods, it can run on mouse or human samples. When using mouse data, the network must always be provided. CARNIVAL produces a set of networks that can be directly visualised. A pathway enrichment analysis can be run over the results. Then, these data can be visualised on bar and volcano plots. Please find here the Tutorial.
COSMOS
COSMOS is a tool to integrate multiomic data with a prior knowledge network spanning signaling, gene regulation and metabolism ( Dugourd et al. 2021 ). It uses the ILP formulation of CARNIVAL to connect two sets of upstream and downstream molecular features (e.i. kinase activities, TF activities, deregulated metabolites, enzyme fluxes, genetic or drug perturbations, etc…) with a signed directed transomic network. This resulting network is essentially a set of coherent mechanistic hypotheses that can explain how the measured deregulation may explain each other. Subsets of this network centered on user-defined nodes can be viewed in the shiny app. The network can also be downloaded as a pair of sif and attribute csv files. These files can be imported in tools such as cytoscape to visualise the full network. Please find here the Tutorial.